[ad_1]
Four eye ointment products sold in some Walmart and CVS stores have been recalled because the facility in which they were manufactured in India may have been unsanitary.
In a recall announcement posted Monday on the Food and Drug Administration website, Brassica Pharma Pvt. Ltd., a product development and manufacturing company in the Indian state of Maharashtra, said an FDA inspection had noted a “lack of sterility assurance at the facility.”
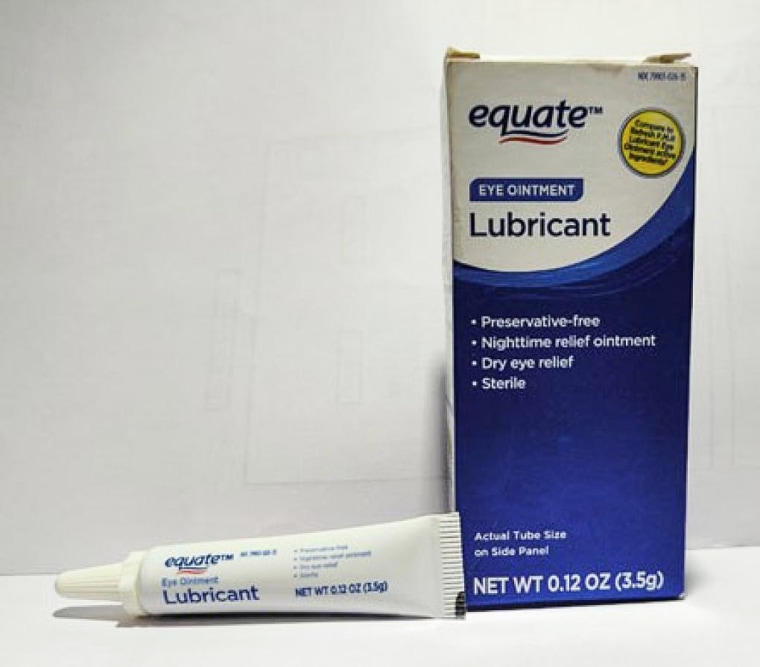
The affected products include two ointments sold under the Equate brand name — Lubricant Eye Ointment and Stye Lubricant Eye Ointment — as well as CVS Health Lubricant Eye Ointment and AACE Pharmaceuticals Lubricant PM Ointment.
“For those patients who use these products, there is a potential risk of eye infections or related harm. These products are intended to be sterile,” the announcement said. “Ophthalmic drug products pose a potential heightened risk of harm to users because drugs applied to the eyes bypass some of the body’s natural defenses.”
The announcement did not specify which types of infections users may be at risk of contracting, nor did it give details about the particular issues at the manufacturing facility. Brassica Pharma did not respond to requests for further information or comment.
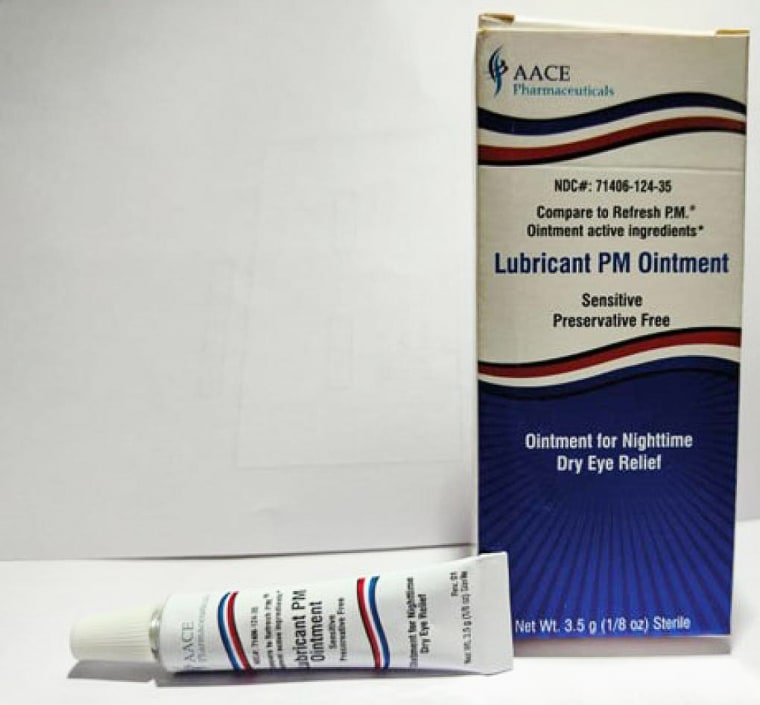
The recalled products are intended to relieve eye dryness or discomfort due to itching, burning, stinging or exposure to wind or sun.
Brassica Pharma had not received any reports of infection or other injuries related to the recalled ointments as of Feb. 16, according to the recall announcement. But the company said that consumers should stop using the recalled products and that they can return them to the stores where they were purchased.
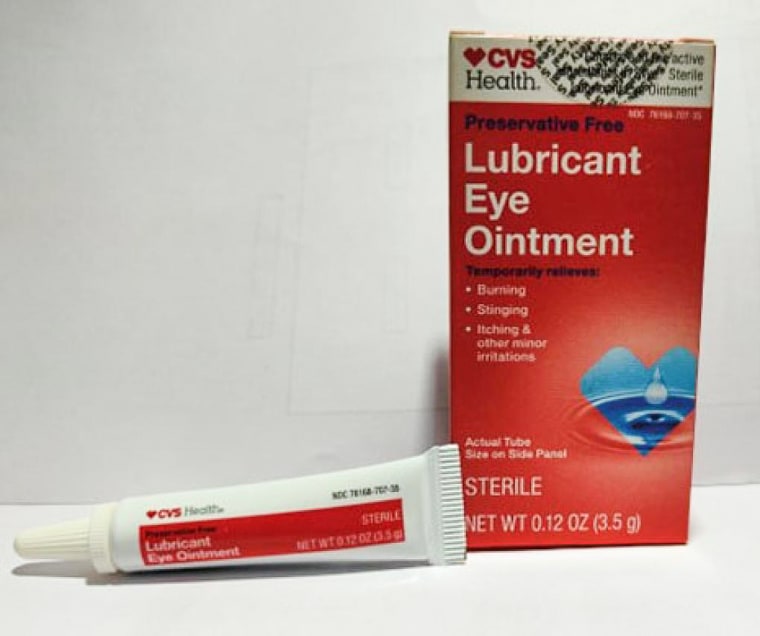
The recalled products have expiration dates between February 2024 and September 2025, according to the announcement, and are sold in 3.5-gram tubes that come packaged in cardboard boxes.
The recall came after a string of high-profile incidents in which users contracted serious eye infections from contaminated eyedrops. By May, 81 people had been diagnosed with drug-resistant bacterial infections after having used contaminated eyedrops. Among those cases, 14 people were blinded, four others had to have their eyeballs surgically removed, and four people died. Many affected patients had used drops from the brands EzriCare and Delsam Pharma; those products were recalled.
In August, the FDA told consumers not to use two other types of eyedrops because of a risk of fungal and bacterial contamination. Then the agency issued a warning in November about 28 other eyedrop products sold by Target, Rite Aid, CVS and other retailers. Several of the listed items, some from the brands Leader, Rugby Laboratories and Kilitch Healthcare India Limited, have since been recalled.
If you experience problems after using any of the newly recalled eye ointments, contact your health care provider, Brassica Pharma’s announcement says. People can also submit reports about problems caused by the ointments to the FDA’s adverse event reporting program.



